Systems Analysis of Phenotypic Switch in Control of Cancer Invasion
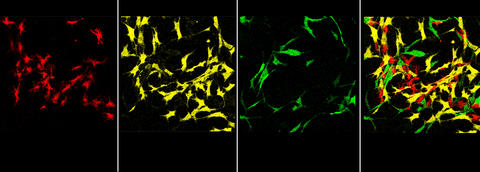
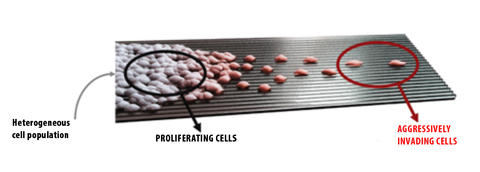
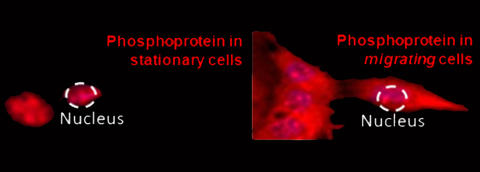
Over 90% of cancer related mortality is linked to invasive and metastatic spread of cancer cells from the primary tumor. In spite of the crucial importance of invasive cancer phenotype, we still have only fragmentary knowledge and understanding of the mechanisms leading to transition from proliferative to aggressive, migratory behavior of cancer cells, which we refer to as Proliferative-to-Aggressive phenotype switching. Increasing evidence suggests that this switch is a reflection of the inherent capacity of cancer cells to adopt both proliferative and migratory phenotypes, with the probability and rate of switching between these two phenotypes controlled by the cell genome, environmental conditions, and cell-cell interactions.
Cancer Systems Biology at Yale (CaSB@Yale) addresses the problem of regulation of invasive cancer spread and, more specifically, the Proliferative-to-Aggressive phenotypic switch. A combination of diverse expertise and innovative methods, ranging from synthetic biology, micro- and nano-fabrication technology, evolutionary biology, and mathematical analysis of intracellular molecular networks to the creation of novel CRISPR-based animal models and the design of novel kinase inhibitors that can lay the basis for novel therapeutic compounds are used.
CaSB@Yale brings together researchers from Yale’s Faculty of Arts and Sciences, Yale School of Medicine, Yale School of Engineering & Applied Science, and from Emory University. Investigators work in close collaboration across Yale Systems Biology Institute, Yale Cancer Biology Institute, Yale Cancer Center, Yale skin cancer SPORE, and Yale Neurosurgery department. The work at CaSB@Yale is based on the tightly knit two Research Projects and two support shared resource Cores, focused on the analysis of glioblastoma and melanoma cells, and normal cells of various species modeling invasive growth behavior and phenotypic switching.
The work is supported by the Administrative Core and the results disseminated through various mechanisms mediated by the Outreach and Education Core. The orthogonal and unconventional approaches, characteristic of the highly collaborative use of cutting edge, innovative approaches, will provide an opportunity to advance our understanding of the molecular networks controlling invasive, aggressive cancer spread and lead to new approaches to controlling and treating highly invasive and metastatic malignancies.